Science
The Success of IO Drugs
Immuno Oncology (IO)
Immuno oncology (IO) is a new area of cancer research which has been successful in treating previously untreatable cancers. IO targets the immune system rather than the cancer directly which potentially allows a single IO drug to be used to treat multiple cancer types with mostly manageable side effects.

Checkpoint Inhibitors
Checkpoint inhibitors (anti-PD-1/PD-L1 or anti-CTLA-4) are clinically validated cancer
treatments and although they have remarkable efficacy in some cancers, there is still a large patient population that they cannot treat (ref 1).

Response Rate
The current response rate of checkpoint inhibitors is estimated to be 15-60% depending on the cancer type, leaving the majority of patients without a treatment option (ref 2). Further, approximately 1/3 patients relapse, indicating the
development of resistance (ref 3).

Myeloid Cells
An important class of immune cells, called myeloid cells, including myeloid derived suppressor cells (MDSCs), tumor associated macrophages and immature dendritic cells (DCs), represent alternative drug target populations that accumulate in cancer and can undermine the efficacy of current cancer treatments (ref 4 and 5).

The Next Wave
We believe that novel drugs targeting these suppressive myeloid cells will be the next wave of successful IO treatments has the potential to unlock the power of IO to more patients than is currently possible.
1. Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350-1355, doi:10.1126/science.aar4060 (2018).
2. Das, S. & Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306. doi: 10.1186/s40425-019-0805-8.
3. Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350-1355, doi:10.1126/science.aar4060 (2018).
4. Wilcox, R. A. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clinic proceedings 85, 656-663, doi:10.4065/mcp.2010.0077 (2010).
5. Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII 58, 49-59, doi:10.1007/s00262-008-0523-4 (2009)
TECHNOLOGY
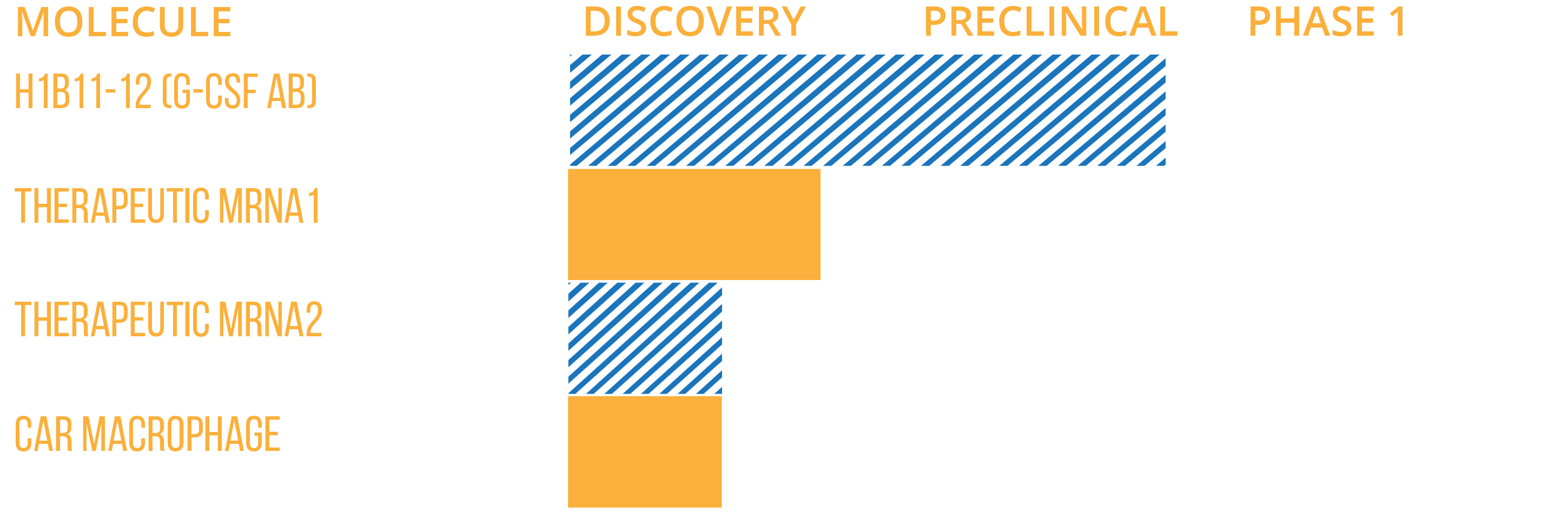
ME Therapeutics has two myeloid cell targeted drug development programs and one drug discovery program currently underway. All three programs target distinct areas of myeloid cell biology in order to inhibit the suppressive effects of suppressive myeloid cells on the anti-cancer immune response. These drug candidates are being developed to target pathways of myeloid cell biology that are not currently being targeted effectively.
Drug Development
Our two development programs include our anti-G-CSF antibody candidate (h1B11-12) and our myeloid targeted prodrug candidates (D094 and D099).
Drug DISCOVERY
Our drug discovery program is focussed on the discovery of novel lipid nanoparticle formulations capable of effectively delivering small molecule drugs and or nucleic acids to myeloid cells in tumors. This program will support our existing prodrug development as well as potentially provide therapies for targeting myeloid cells in IO.
RESEARCH SHOWS
Combination Therapies Can Improve Patient Outcome. Current IO drugs (especially checkpoint inhibitors) have shown remarkable efficacy in some patients:
- Most cancer types are believed to be amenable to IO
- IO targets the immune system and not cancer directly
- Not all patients respond to single-agent IO
- Large market (estimated $154B by 2030)*
- Combining checkpoint inhibitors with myeloid cell targeting therapy has potential increase response rate
- Allow more patients to benefit from IO
- Target large unmet need
- Increase market share for existing therapies through more effective combinations
* Spherical Insights
The promise for immunotherapy oncology
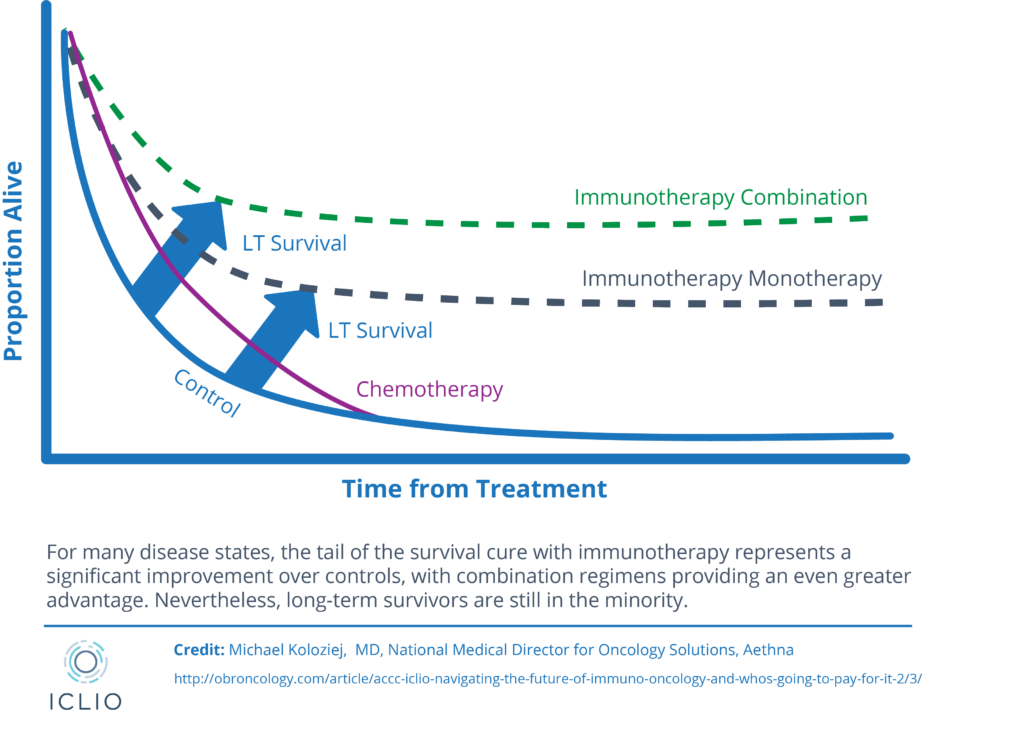
MYELOID CELLS HINDER CANCER KILLING

Myeloid Cells are a roadblock to immune checkpoints and hinder cancer killing

Checkpoint inhibitors focus on activating cancer killing T cells (Keytruda, Opdivo, Yervoy)

Myeloid cells interfere with T cell function but can be targeted or reprogrammed to help eliminate tumor cells

We believe that combining myeloid targeting with T cell targeting will be key in next generation IO treatments
Myeloid Targeted Pro-drug Candidates
D094 and D099 are small molecule prodrug candidates under development to treat cancer.
Composed of an active small molecule drug linked to 2 different lipids and inserted into lipid nanoparticles (LNPs).
Designed to preferentially release the active drug in the tumour microenvironment.
The active drug component has been shown to block a key suppressive pathway in myeloid cell biology and directly kill some tumour cells.
G-CSF Antibody Candidate Development
h1B11-12, a humanized antibody candidate targeting a potential key player in cancer induced immune suppression by myeloid cells.
h1B11-12 is a biological drug which works by targeting and blocking a cytokine (immune system protein) called G-CSF.
G-CSF is a glycoprotein cytokine that is low or undetectable in healthy individuals but is transiently induced during acute inflammation.
Sustained G-CSF production in tumours plays a critical role in promoting MDSC production and restricting DC maturation leading to immunosuppression (references).
THE FACTS
Cancer is on the rise. Research suggests we can expect over 29.5 million new cases of cancer by 2040.
Lung Cancer
238,340 estimated cases in the U.S. in 2023
BREAST Cancer
297,790 estimated
cases in the U.S.
in 2023

COLORECTAL Cancer
153,020 estimated
cases in the U.S.
in 2023
OUR INTELLECTUAL PROPERTY
PCT filed February 2018 on composition and use of lead anti-G-CSF antibodies (PCT/CA2018/050143) – Chinese patent granted in 2023.
Patents on prodrugs are expected to be filed once testing is complete – Initial search suggests freedom to operate based on proposed drug structures.
Pipeline